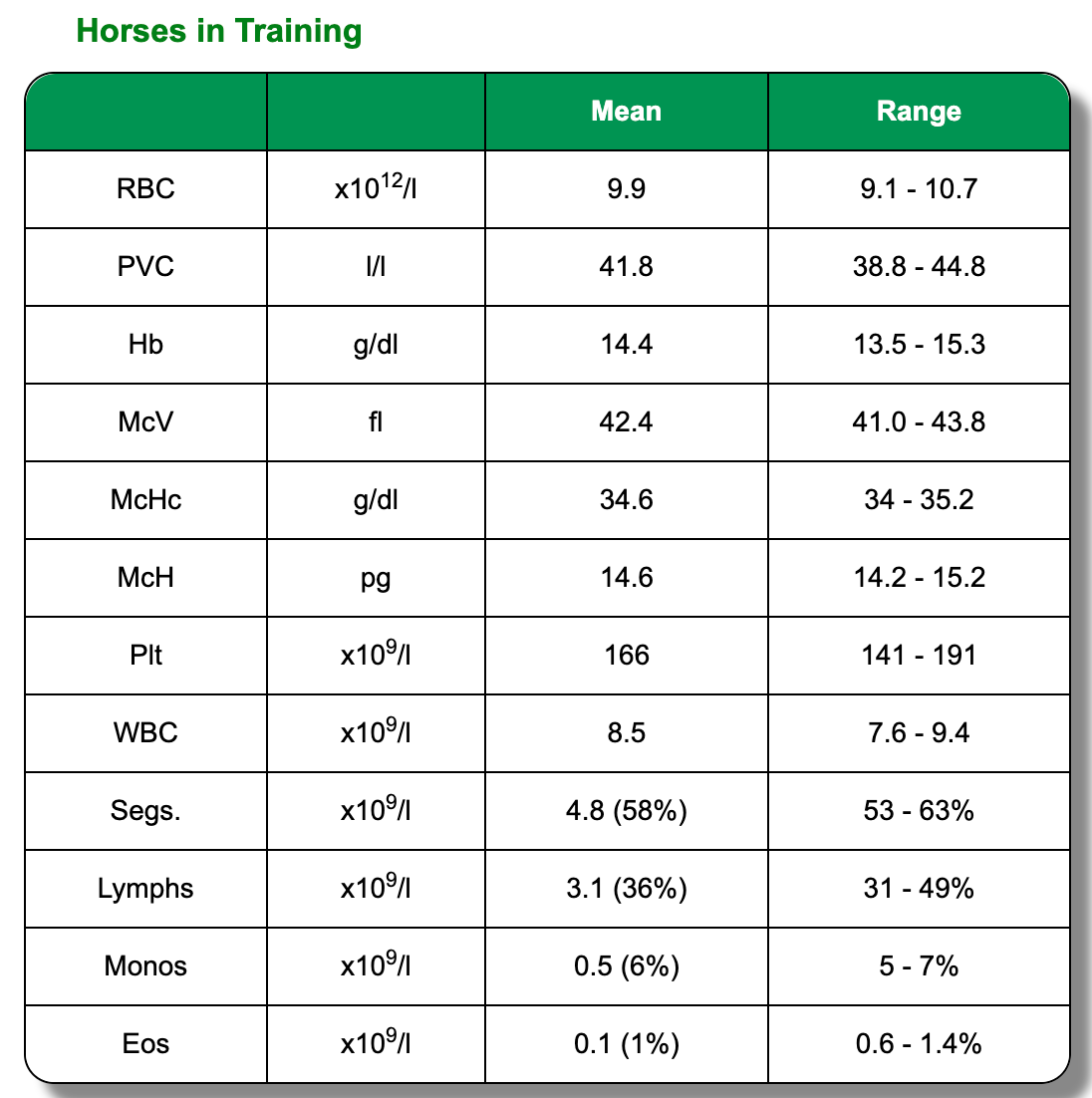
Red Blood Cells (RBC)
Every undergraduate and many laypersons understand that Packed Cell Volume (PCV l/l or %) rises in dehydration and shock and falls in (extreme) haemorrhage. PCV is however, probably the least useful RBC measure in the elite athlete. As noted previously, it is a labile parameter influenced by splenic contraction following exercise or excitement and blood biochemistry is a better means of evaluating hydration status. Identification of the anaemia that is associated with chronic infection is the entity that is of most importance in evaluating RBC criteria in the elite equine athlete. From a practical point of view, anaemia can be suspected where Haemoglobin (Hb g/l) values are < 12 for thoroughbreds and 11 for sport horses. Care must be
exercised here however, as older thoroughbreds e.g. mature National Hunt / jump racing horses can be so relaxed on sampling that their Hb values can be misleadingly low. Equine erythrocytes do not follow the patterns associated with regeneration in other species. Anisocytosis and reticulocytosis are only seen, in the authors experience, in the most extreme and long-standing anaemia. Serial evaluation of RBC, Hb and total protein are probably the best way to follow a post- anaemic erythropoetic response.
White Blood Cells (WBC)
In elite horses leucocytosis and leucopaenia can be defined as WBC > 10.0 x 109/ L
and < 6.0 x 109/ L respectively. Values lying close to these values should be interpreted with caution given the potentially confusing effects of catecholamine induced mobilisation of cells in this series and the need for appropriate filling of EDTA tubes. Failure to fill correctly can result in an incorrect coagulant to blood ratio with consequently misleading WBC values. Evaluation of the individual components of the WBC series is usually much more informative than reliance on WBC counts in isolation, hence the need for EDTA samples, rather than lithium heparin for Haematology as referred to previously.
Neutrophils / Polymorphonuclear cells (PMNL)
PMNL’s are marginated in numbers on the endothelium of the peripheral blood vessels and are released into the circulation in response to stress, infection and inflammation. PMNL values are often reported as the percentage of Neutrophils (N%) in a differential cell count. When reported in this way, the Neutrophil:Lymphocyte
ratio in mature adult elite horses is somewhere around a 60%:40% ratio, respectively. Neutrophilia and neutropaenia are better identified by recourse to absolute PMNL counts. Neutrophilia, when accompanied by hyperfibrinogenaemia, is usually noteworthy when PMNL’s are > 7.0 x 109 / L. It is impossible to meaningfully differentiate between the neutrophilia of stress and that of infection and inflammation, without concurrent measure of acute phase proteins such as
fibrinogen. Neutropaenia can be defined as PMNL’s < 2.0 x 10 9 / L, when they can be a very disturbing indicator of impending catastrophies, e.g. colitis / laminitis / peritonitis, etc. Immature neutrophils, whether toxic or band form, are associated with the presence of pus-forming infections and are best evaluated using manual
differential counts derived from fresh blood smears.
Lymphocytes
Lymphocytosis (Lymphs > 5.0 x 109 / L) is a feature of chronic bacterial infections
and the secondary phase of bacterial infection that often follows from primary viral
infections. Lymphopaenia (Lymphs < 1.0 x 10 9 / L) may be seen in septic shock and
severe viral infections and although reported as such, is seldom of much use in the
authors experience in the identification of lymphosarcoma.
Monocytes
Monocytes are macrophage precursors. Monocytosis (Monocytes > 0.5 x 109 / L) can
reflect cellular responses to chronic bacterial infections.
Eosinophils
Eosinophilia (Eosinophils > 0.5 x 109 / L) can be seen in parasitism and allergy. It may also be seen in chronic eosinophilic granulomatous enteritis.