- +353 (0)45 866266

General screening for subclinical and some clinical entities in elite equine athletes is often undertaken using a battery or package of tests that are chosen by the laboratory or by the individual clinician. Low-grade abnormalities or significant clinical entities can then be further investigated, by study of specific organ systems e.g. liver, kidney, GIT etc. This approach has much to recommend it, but it is important to remember that the statistical probability of having one or more values outside a reference range increases can increase with the number of tests carried out, regardless of the presence of disease or other compromise. Reference ranges for frequently used biochemical tests such as total protein and globulin can be based on selection of horses with normal WBC and plasma fibrinogen values.
Additional Information:
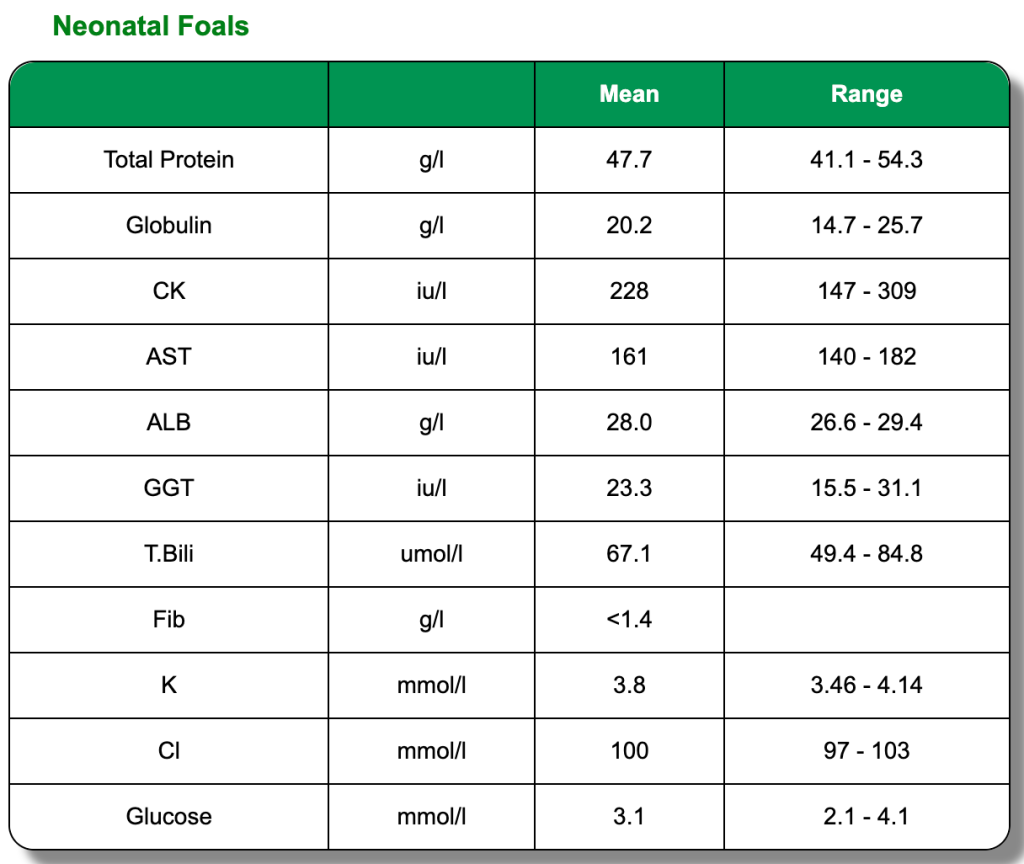
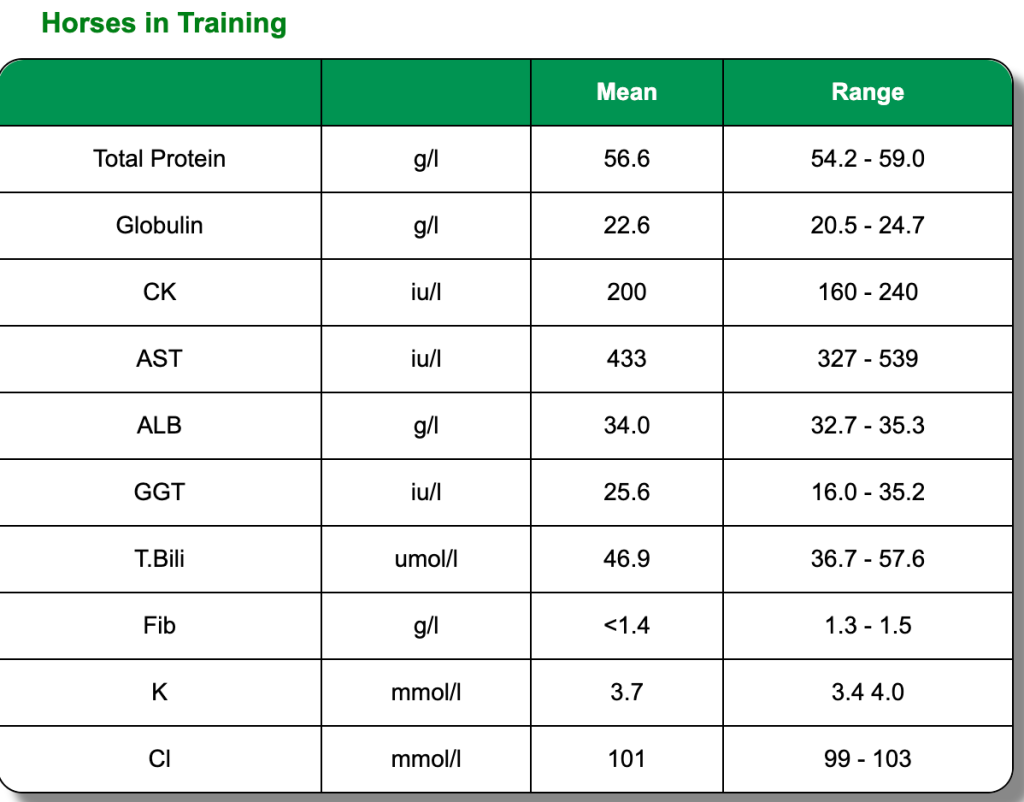
The below clinical pathology reference tables are a general guide. Each horse is an individual and will have its own norms.These reference tables are based on Thoroughbred foals and two or three year old horses in training. All were in good health and condition on clinical examination. Click on each test to learn more or go directly to the biochemistry blood page.
Sign up for our newsletter and keep up to date with industry news!
Sign up for our newsletter and keep up to date with industry news!
Privacy Policy | Terms & Conditions
© Copyright Irish Equine Center. All Rights Reserved | Designed by DMC Consultancy LTD